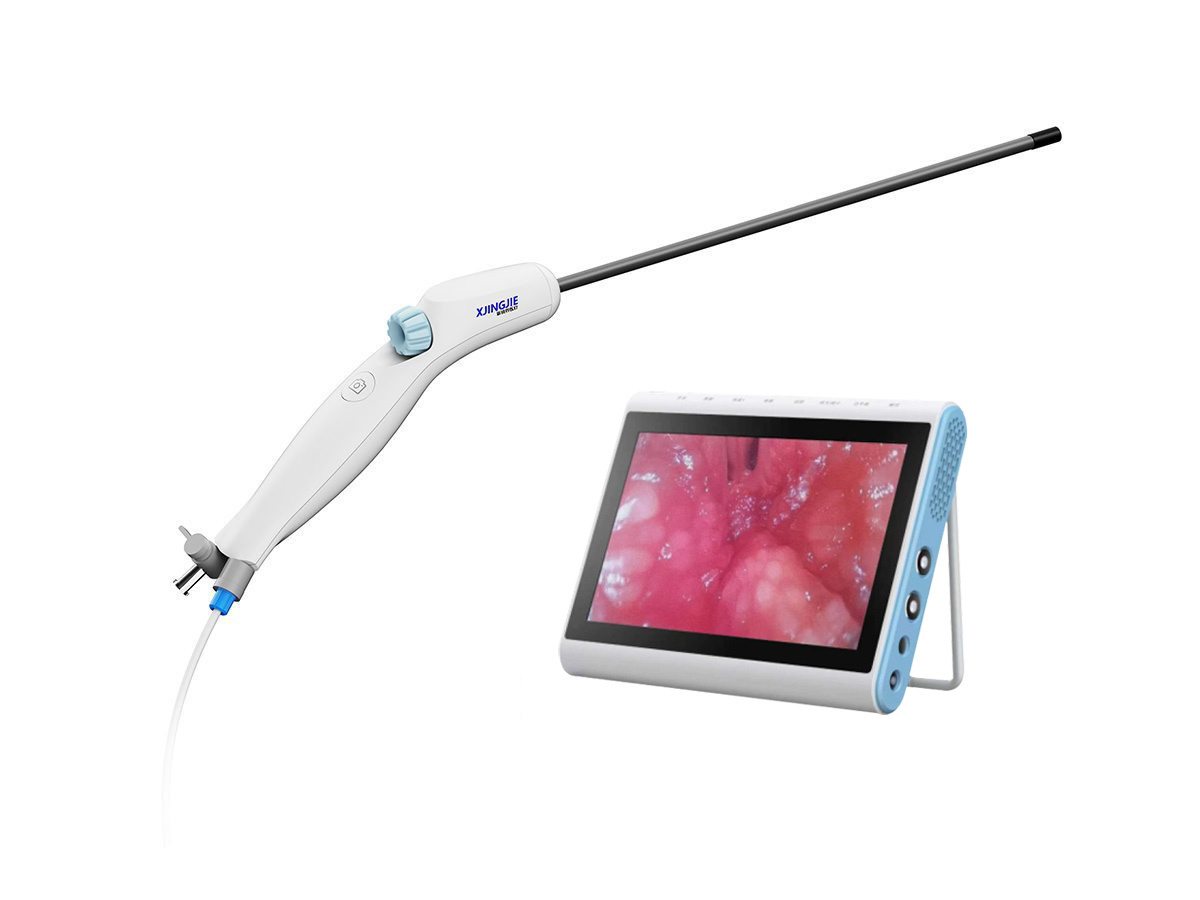
Single-use bronchial intubation
Xiang Medical Device Registration Approval 20222080479
- Commodity name: Single-use bronchial intubation
- 编号: Xiang Medical Device Registration Approval 20222080479
Key words:
Key words:
Single-use bronchial intubation
Product Consulting
Contact Us
Phone:0731-52326039
Email:xjingjie@xjingjie.com.cn
hndee@hndee.com.cn
Address: No. 31 Dongfeng Road, Xiangtan Economic Development Zone, Innovation and Entrepreneurship Center, Building 12, 4th Floor
Business License